A gas phase decomposition reaction
2AB3 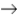 A2 + 3B2
A2 + 3B2
Is exothermic. What can you conclude about the spontaneity of this reaction?
A) It is non-spontaneous.
B) Nothing conclusive can be stated without more information.
C) It is spontaneous.
Correct Answer:
Verified
Q129: A 1.00-mol sample of an ideal monatomic
Q130: For the reaction CO(g) + 2H2(g)
Q131: Given that S° = 131 J/Kmol for
Q132: In the equation P1V1

Q133: In which of the following changes is
Q135: Identify the second law of thermodynamics.
A) In
Q136: For a certain process, at 311 K,
Q137: For a certain process, at 319 K,
Q138: For the process A(l) → A(g), ΔH°
Q139: One mole of an ideal gas is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents