Consider the following reaction: 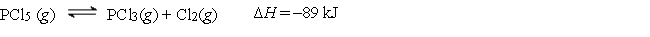
-How can the equilibrium be shifted to the right?
A) Remove Cl2.
B) Decrease the pressure by changing the volume.
C) Remove PCl3.
D) Add more PCl5.
E) Any of these will shift the equilibrium to the right.
Correct Answer:
Verified
Q44: Raising the pressure by lowering the volume
Q45: Consider the following equilibrium: Q46: Consider the following equilibrium:N2(g) + 3H2(g) Q47: Consider the equation 2A(g) Q48: Consider the following equilibrium:N2(g) + 3H2(g) Q50: Consider the following equilibrium:N2(g) + 3H2(g) Q51: To increase the value of K for Q52: Consider the equation 2A(g) Q53: When the substances in the equation below Q54: For a certain reaction at 25.0°C, the Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()

