Consider the following chemical reaction involving a pure solid sample A that yields products B and C:
3A(s) 
3B(g) + 2C(g)
Identify the equilibrium expression of the reaction.
A) 
B) 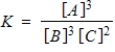
C) 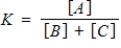
D) 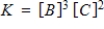
E) 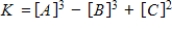
Correct Answer:
Verified
Q58: Explain how chemical equilibrium is microscopically dynamic
Q59: Consider the following reaction (assume an ideal
Q60: Given the reaction A(g) + B(g)
Q61: To achieve equilibrium, the original reaction mixture
Q62: Derive the relationship between K and Kp.
Q64: At room temperature cyclohexane exists almost exclusively
Q65: Which of the following determines the equilibrium
Q66: Write the equilibrium constant expression for the
Q67: Once equilibrium was established, some additional chlorine
Q68: For a chemical reaction system, Q and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents