Samples of the gases H2(g) and SO2(g) have equal masses and are at the same temperature and pressure. Calculate the following:
The ratio of volumes 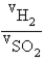
A) 32
B) 0.18
C) 180
D) 5.6
E) 1.0
Correct Answer:
Verified
Q5: A balloon contains 10.0 g of neon
Q6: Which of the following relationships is not
Q7: A sample of oxygen gas has a
Q8: A cylinder is fitted with a movable
Q9: Mercury vapor contains Hg atoms. What is
Q11: Body temperature is about 308 K. On
Q12: Three 1.00-L flasks at 25°C and 725
Q13: Given a cylinder of fixed volume filled
Q14: Consider two samples of helium in separate
Q15: The valve between a 3.00-L tank containing
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents