Samples of the gases H2(g) and SO2(g) have equal masses and are at the same temperature and pressure. Calculate the following:
-The ratio of the root-mean-square velocities 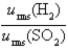 .
.
A) 180
B) 32
C) 0.18
D) 1.0
E) 5.6
Correct Answer:
Verified
Q74: Under which of the following conditions does
Q75: Calculate the temperature at which the average
Q76: Calculate the following ratios for a gas
Q77: Consider separate samples of Ar(g) and Ne(g).
Q78: Calculate the ratio of the change in
Q80: Consider two 1.0-L containers, one containing He(g)
Q81: Samples of the gases H2(g) and SO2(g)
Q82: Volume versus temperature in degrees Celsius for
Q83: The ratio V/n versus the Kelvin temperature
Q84: Which gas, He or H2O vapor, has
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents