The atomic mass of rhenium is 186.2. Given that 37.1% of natural rhenium is rhenium-185, what is the other stable isotope?
A) 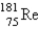
B) 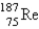
C) 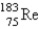
D) 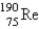
E) 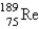
Correct Answer:
Verified
Q13: Calculate the molar mass of a sample
Q14: Iron is biologically important in the transport
Q15: A single atom of an element weighs
Q16: For a new element, 60.54% is an
Q17: The average mass of a boron atom
Q19: An alkali metal oxide contains 83.01% metal
Q20: Which compound has the smallest molar mass?
A)
Q21: You find a compound composed only of
Q22: The molar mass of the insecticide dibromoethane
Q23: What is the percent by mass of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents