For the question(s) that follow, consider the following balanced equation.
Mg₃N2(s) + 6H2O(l) → 3Mg (OH) ₂(s) + 2NH₃(g)
-What is the correct form of the conversion factor needed to convert the number of moles of H2O to the number of moles of NH₃ produced?
A) 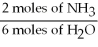
B) 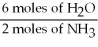
C) 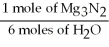
D) 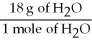
E) 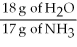
Correct Answer:
Verified
Q43: What is the molar mass of Mg₃(PO₄)₂
Q44: In the reaction of nitrogen gas, N2,
Q45: One mole of neon atoms has a
Q46: For the question(s) that follow, consider the
Q47: For the question(s) that follow, consider the
Q49: Given the following equation, what is the
Q50: For the question(s) that follow, consider the
Q51: 1.25 moles of PbO2 have a mass
Q52: The molar mass of calcium hydroxide, Ca(OH)₂
Q53: For the following question(s),consider the following equation.
2Mg
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents