What is the correct form of the equilibrium constant for this reaction? 2H2O2 (g) ⇌ 2H2O (g) + O2 (g)
A) 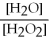
B) 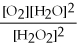
C) 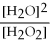
D) 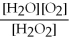
Correct Answer:
Verified
Q40: For the following reaction, the equilibrium constant
Q41: The equilibrium between hemoglobin and oxyhemoglobin in
Q42: 2SO2(g) + O2(g) ⇌ 2SO₃(g) For the
Q42: The rule or principle that describes the
Q43: A catalyst for a chemical reaction affects
Q44: The rate of a chemical reaction depends
Q47: The equilibrium constant is the ratio of
Q48: A catalyst lowers the activation energy of
Q49: Write the equilibrium constant expression for the
Q50: Activation energy is always a large amount
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents