For the following reaction, the equilibrium constant Kc is 2.0 at a certain temperature. Write the equilibrium constant expression of the equilibrium constant, Kc. 2NOBr(g) ⇌ 2NO(g) + Br2(g)
A) 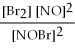
B) 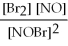
C) 
D) 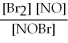
E) 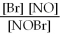
Correct Answer:
Verified
Q47: The equilibrium constant is the ratio of
Q48: A catalyst lowers the activation energy of
Q49: Write the equilibrium constant expression for the
Q50: Activation energy is always a large amount
Q51: One of the substances acted upon by
Q53: For the following reaction, the equilibrium constant
Q54: At equilibrium, the concentrations of the reactants
Q55: For the following reaction, the equilibrium constant
Q57: The rate of a chemical reaction is
Q79: The _ is the energy difference between
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents