The electron states in an atom which constitute a single subshell all have:
A) only the same value of n
B) only the same value of 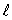
C) only the same value of n and the same value of 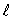
D) only the same value of 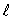 and the same value of
and the same value of 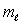
E) the same set of all four quantum numbers
Correct Answer:
Verified
Q13: An atom is in a state with
Q14: The magnitude of the orbital angular momentum
Q15: An electron in an atom is in
Q16: An electron in an atom is
Q17: In the relation Q19: Space quantization means that: Q20: The possible values for the magnetic Q22: The minimum energy principle tells us that: Q23: Which of the following Q37: The Pauli exclusion principle is obeyed by:![]()
A) space is quantized
B)
A)![]()
A)all
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents