When a lithium atom is made from a helium atom by adding a proton (and neutron) to the nucleus and an electron outside, the electron goes into an n = 2, 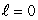 state rather than an n = 1,
state rather than an n = 1, 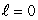 state. This is an indication that electrons:
state. This is an indication that electrons:
A) obey the Pauli exclusion principle
B) obey the minimum energy principle
C) undergo the Zeeman effect
D) are diffracted
E) and protons are interchangeable
Correct Answer:
Verified
Q25: The Stern-Gerlach experiment makes use of:
A)a strong
Q26: Electrons are in a two-dimensional square potential
Q27: Six electrons are in a two-dimensional square
Q28: When a lithium atom in its ground
Q30: Five electrons are in a two-dimensional square
Q32: No state in an atom can be
Q32: If electrons did not have intrinsic angular
Q34: Five electrons are in a two-dimensional square
Q35: Electrons are in a two-dimensional square potential
Q36: The force exerted on a magnetic dipole
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents