The most energetic electron in any atom at the beginning of a period of the periodic table is in:
A) an 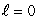 state
state
B) an 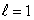 state
state
C) an 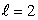 state
state
D) an n = 0 state with unspecified angular momentum
E) an n = 1 state with unspecified angular momentum
Correct Answer:
Verified
Q49: An electron in an L shell
Q54: The group of atoms at the beginning
Q56: The K
Q57: The effective charge acting on a single
Q58: The states being filled from the beginning
Q60: The ionization energy of an atom
Q63: In a helium-neon laser, the laser light
Q68: Which of the following is essential for
Q72: A laser must be pumped to achieve:
A)a
Q76: In calculating the x-ray energy levels the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents