An ideal gas is to taken reversibly from state i, at temperature T1, to another states labeled I, II, III, IV and V on the p-V diargram below. All are at the same temperature T2. Rank the five processes according to the change in entropy of the gas, least to greatest. 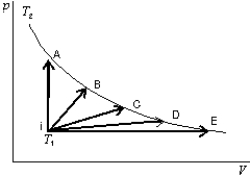
A) I, II, III, IV, V
B) V, IV, III, II, I
C) I, then II, III, Iv, and V tied
D) I, II, III, and IV, tied, then V
E) I and V tied, then II, III, IV
Correct Answer:
Verified
Q4: In a reversible process the system:
A)is always
Q5: Which of the following processes leads to
Q6: An ideal gas expands into a vacuum
Q6: Possible units of entropy are:
A) J
B) J/K
C)
Q7: Rank from smallest to largest, the changes
Q8: Consider the following processes: The temperature of
Q10: A hot object and a cold
Q11: The temperature of n moles of a
Q15: For all adiabatic processes:
A)the entropy does not
Q17: The difference in entropy
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents