The pressure of an ideal gas is doubled in an isothermal process. The root-mean-square speed of the molecules:
A) does not change
B) 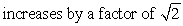
C) 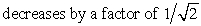
D) increases by a factor of 2
E) decreases by a factor of 1/2
Correct Answer:
Verified
Q9: It is known that 28 grams of
Q12: An ideal gas undergoes an isothermal process
Q20: In order that a single process be
Q28: A certain ideal gas has a temperature
Q40: When an ideal gas undergoes a slow
Q48: In a certain gas the molecules are
Q52: The temperature of a gas is most
Q55: The mean free path of molecules in
Q57: The mean free path of a gas
Q60: The mean free path of molecules in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents