A sample of argon gas (molar mass 40 g) is at four times the absolute temperature of a sample of hydrogen gas (molar mass 2 g) . The ratio of the rms speed of the argon molecules to that of the hydrogen is:
A) 1
B) 5
C) 1/5
D) 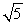
E) 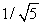
Correct Answer:
Verified
Q29: The speeds of 25 molecules are distributed
Q47: The temperature of low pressure hydrogen
Q51: The Maxwellian speed distribution provides a direct
Q53: Evidence that molecules of a gas are
Q54: The average speed of air molecules at
Q55: Five molecules have speeds of 2.8, 3.2,
Q64: According to the Maxwellian speed distribution, as
Q71: As the volume of an ideal gas
Q75: Ideal monatomic gas A is composed of
Q79: For a gas at thermal equilibrium the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents