The electron states in an atom which constitute a single shell all have:
A) the same value of n
B) the same value of 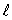
C) the same value of n and the same value of 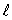
D) the same value of 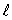 and the same value of
and the same value of 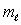
E) the same set of all four quantum numbers
Correct Answer:
Verified
Q2: Possible values of the principal quantum
Q3: The electron states in an atom which
Q4: The number of states in a shell
Q4: The number of possible values of the
Q5: The number of states in a subshell
Q9: The possible values for the magnetic
Q10: An electron in an atom is in
Q11: An electron in an atom is
Q12: The magnitude of the orbital angular momentum
Q20: The quantum number ms is most closely
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents