The most energetic electron in any atom at the beginning of a period of the periodic table is in:
A) an 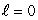 state
state
B) an 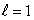 state
state
C) an 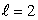 state
state
D) an n = 0 state with unspecified angular momentum
E) an n = 1 state with unspecified angular momentum
Correct Answer:
Verified
Q42: An electron in a K shell
Q42: Suppose the energy required to ionize an
Q44: In connection with x-ray emission the
Q48: The group of atoms at the ends
Q49: The most energetic electron in any atom
Q50: Characteristic K x-radiation of an element is
Q52: In connection with x-ray emission the
Q54: The group of atoms at the beginning
Q56: The K
Q64: In a Moseley graph:
A)the x-ray frequency is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents