The diagram shows three isotherms for an ideal gas, with T3-T2 the same as T2-T1. It also shows five thermodynamic processes caried out on the gas. Rank the processes in order change in the internal energy of the gas, least to greatest. 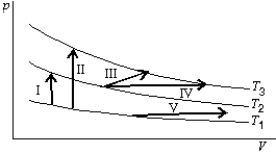
A) I, II, III, IV, V
B) V, then I, III, and IV tied, then II
C) V, I, then I, III, and IV tied, then II
D) IV, V, III, I, II
E) II, I, then III, IV, and V tied
Correct Answer:
Verified
Q1: Evidence that a gas consists mostly of
Q22: Air is pumped into a bicycle tire
Q27: According to the kinetic theory of gases,
Q30: The mean free path of air molecules
Q35: The force on the walls of a
Q39: The pressure of an ideal gas is
Q46: The root-mean-square sped of molecules in a
Q51: The Maxwellian speed distribution provides a direct
Q54: If the temperature T of an ideal
Q55: The mean free path of molecules in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents