A sample of argon gas (molar mass 40 g) is at four times the absolute temperature of a sample of hydrogen gas (molar mass 2 g) . The ratio of the rms speed of the argon molecules to that of the hydrogen is:
A) 1
B) 5
C) 1/5
D) 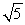
E) 
Correct Answer:
Verified
Q29: The speeds of 25 molecules are distributed
Q43: If the molecules in a tank of
Q53: Evidence that molecules of a gas are
Q54: The average speed of air molecules at
Q61: According to the Maxwellian speed distribution, as
Q67: As the pressure in an ideal gas
Q71: As the volume of an ideal gas
Q79: For a gas at thermal equilibrium the
Q87: The number of degrees of freedom of
Q96: An ideal gas has molar specific
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents