The figure shows the energy levels for an electron in a finite potential energy well. If an electron in the n = 2 state absorbs a photon of wavelength 2.0 nm, what happens to the electron? 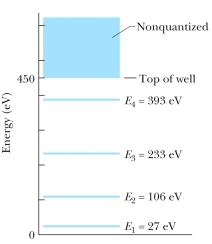
A) It makes a transition to the n = 3 state.
B) It makes a transition to the n = 4 state.
C) It escapes the well with a kinetic energy of 280 eV.
D) It escapes the well with a kinetic energy of 730 eV.
E) Nothing; this photon does not have an energy corresponding to an allowed transition so it is not absorbed.
Correct Answer:
Verified
Q22: The series limit for the Balmer
Q23: Take the potential energy of a hydrogen
Q30: Consider the following: Q31: A particle in a certain finite potential Q32: A particle is trapped in a finite Q33: When a hydrogen atom makes the transition Q35: Take the potential energy of a hydrogen Q36: The diagram shows the energy levels for Q37: Take the potential energy of a hydrogen Q42: The radial probability density for the electron![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents