Which molecule(s) is/are likely to be a gas at room temperature due to a lack of attraction to another molecule of its own kind? 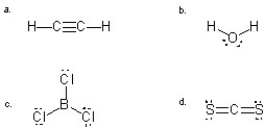
A) a,b,and c
B) a,c,and d
C) b
D) c and d
Correct Answer:
Verified
Q18: A oxygen atom with one bond and
Q19: A neutral sulfur atom has _ valence
Q20: What is the electronic geometry around the
Q21: Which atom is the MOST electronegative?
A) Ca
B)
Q22: Which structure BEST describes the polarity of
Q24: What is the correct ELECTRONIC GEOMETRY and
Q25: Which molecule has a NET DIPOLE?
Q26: Based on the Pauling electronegativities,an Fr-F bond
Q27: Based on the Pauling electronegativities,a C-H bond
Q28: Which molecule will have the STRONGEST attraction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents