If the accompanying drawing of an electrochemical cell represents an iron-nickel cell,use the partial activity series included to identify the components labeled 1,2,3,and 4 respectively. 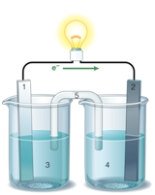 Zn Zn2+ + 2e- Fe Fe2+ + 2e-
Zn Zn2+ + 2e- Fe Fe2+ + 2e-
Co Co2+ + 2e-
Ni Ni2+ + 2e-
Cu Cu2+ + 2e-
A) iron,nickel,iron ion solution,nickel ion solution
B) nickel,iron,iron ion solution,nickel ion solution
C) nickel,iron,nickel ion solution,iron ion solution
D) iron,nickel,nickel ion solution,iron ion solution
Correct Answer:
Verified
Q31: If the accompanying drawing of an
Q32: Which example represents the reduction half-reaction
Q33: The reduction half-reaction for the reaction
Q34: What are the coefficients of the
Q35: The oxidation half-reaction for the reaction
Q37: Given the accompanying partial activity series,which
Q38: Using the accompanying drawing of an electrochemical
Q39: Knowing that sodium and potassium react violently
Q40: Given the accompanying partial activity series,which
Q41: What are the coefficients of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents