In the diagram,the activation energy of the FORWARD reaction is about: 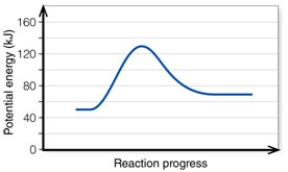
A) 60 kJ.
B) 130 kJ.
C) 80 kJ.
D) 20 kJ.
Correct Answer:
Verified
Q3: In the diagram,the forward reaction will probably
Q4: Which statement correctly describes reaction rates?
A) Decreasing
Q5: In the diagram,the number 5 corresponds to
Q6: Which statement correctly describes reaction rates?
A) Decreasing
Q7: In the diagram,how do the concentrations of
Q9: In the diagram,the energy of the transition
Q10: In the diagram,the number 3 corresponds to
Q11: In the diagram,the reaction described is _
Q12: When a chemical reaction is at equilibrium,which
Q13: In the diagram,the number 2 corresponds to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents