Consider the following table of relative strengths of oxidizing and reducing agents:
Oxidizing Agent
Reducing Agent
Stronger
A+ + e- 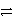
A
Weaker
B2+ + 2 e- 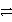
B
Weaker
C3+ + 3 e- 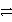
C
Stronger
Which of the following is the correct net ionic equation for the redox reaction between A+ and C and the correct direction that is favored?
A) A+ + C 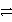 A + C3+ forward
A + C3+ forward
B) A+ + C 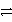 A + C3+ reverse
A + C3+ reverse
C) 3 A+ + C 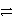 3 A + C3+ forward
3 A + C3+ forward
D) 3 A+ + C 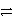 3 A + C3+ reverse
3 A + C3+ reverse
E) A+ + C + e- 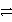 A + C3+ + 3 e- forward
A + C3+ + 3 e- forward
Correct Answer:
Verified
Q20: Which of the following is a reduction
Q23: What is the reducing agent in the
Q23: What role does the reducing agent play
Q24: In the reaction of carbon with oxygen,which
Q27: Which of the following substances is most
Q28: Which of the following is the correct
Q30: When zinc is plated to iron,the zinc
Q30: Which of the following is the correct
Q31: Which of the following statements is incorrect?
A)Acid-base
Q34: In comparing acid-base neutralization reactions and redox
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents