Consider the following reaction coordinate diagram. 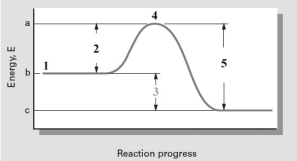 Based on this diagram,which of the following is correct?
Based on this diagram,which of the following is correct?
A) 2 represents E and the reaction is exothermic.
B) 2 represents E and the reaction is endothermic
C) 3 represents E and the reaction is endothermic
D) 3 represents E and the reaction is exothermic
E) 5 represents E and the reaction is exothermic
Correct Answer:
Verified
Q4: Which of the following statements is/are correct?
i.An
Q5: If a reaction is carried out in
Q7: How will the rate of reaction change
Q8: Which of the following conditions is not
Q12: Consider the diagram given below. 
Q13: Consider the hypothetical reaction A + 2
Q14: A molecular collision is sufficiently energetic to
Q14: Consider the following reaction coordinate diagram.
Q15: How does a catalyst alter the rate
Q18: How does increasing the temperature increase the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents