Choose the correct equilibrium constant expression for the equilibrium 2 H2(g) + O2(g)  2 H2O(
2 H2O( 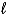 ) .
) .
A) K = 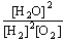
B) K = 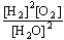
C) K = 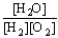
D) K = 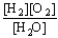
E) K = 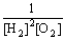
Correct Answer:
Verified
Q26: If the position of equilibrium shifts to
Q32: If an aluminum nitrate solution is added
Q33: Choose the correct equilibrium constant expression for
Q35: Calculate the molar solubility of magnesium fluoride
Q36: Consider the hypothetical reaction A2 + B2
Q38: Predict what will happen if the partial
Q39: What will happen to the position of
Q40: Choose the correct equilibrium constant expression for
Q41: Find the pH of a 0.10 M
Q42: What is the pH of a 0.20
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents