Which of the following is not capable of acting like a Brønsted-Lowry base?
A) H2O( 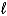 )
)
B) NH4+ ion
C) Cl- ion
D) HNO3(aq)
E) H2PO4-(aq)
Correct Answer:
Verified
Q2: Which of the following is a Brønsted-Lowry
Q5: A Brønsted-Lowry acid is defined as a(n):
A)proton
Q9: What is the conjugate base of water?
A)HCl(aq)
B)H3O+(aq)
C)OH-(aq)
D)H+
Q10: Which of the following is a Lewis
Q13: According to Arrhenius theory,what is an acid?
A)A
Q13: Consider the following generalized reaction. 
Q14: Which pair below cannot have a Brønsted-Lowry
Q15: Which of the following substances is amphoteric?
A)H2O(
Q17: Consider the following image which depicts the
Q20: A substance that can act as both
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents