Which of the following is the correct net ionic equation for the reaction between nitrous acid and hydrogen sulfide ion?
A) HNO2 + HS- 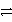 H2NO2+ + S2-
H2NO2+ + S2-
B) HNO2 + HS- 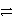 NO2- + H2S
NO2- + H2S
C) HNO2 + HSO4- 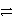 H2NO2+ + SO42-
H2NO2+ + SO42-
D) HNO2 + HSO4- 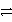 NO2- + H2SO4
NO2- + H2SO4
E) HNO3 + HSO4- 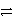 NO3- + H2SO4
NO3- + H2SO4
Correct Answer:
Verified
Q32: A solution is made by dissolving 0.0010
Q33: Which is the correct net ionic equation
Q34: What is the conjugate base of H3C6H5O7?
A)C6H5O73-
B)H2C6H5O7-
C)H3C6H5O7-
D)H4C6H5O7+
E)H3C6H5O7(OH)-
Q35: Given the following acid strengths: HF
Q36: Given the following relative acid strengths,starting with
Q37: Given the following relative base strengths,starting with
Q38: What is the hydroxide ion concentration in
Q39: The hydrogen ion concentration of a solution
Q41: The pH of a solution is 5.330.Find
Q42: Consider the following image. ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents