The structures of cyclohexane and benzene are shown below. 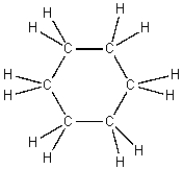
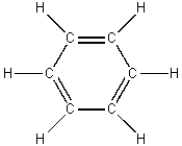
Cyclohexane benzene
It is logical to expect that these liquids are...
A) miscible because of their similar structures and sizes
B) miscible because they contain the same number of carbon atoms
C) immiscible because benzene,with half as many hydrogen atoms,has less hydrogen bonding than cyclohexane
D) immiscible because one molecule is polar and the other nonpolar
E) immiscible because both molecules have ring structures
Correct Answer:
Verified
Q3: What volume of 0.372 M H2SO4 contains
Q4: When a saturated solution is in equilibrium
Q7: Stirring a solute into a solution increases
Q8: Consider the following beaker which has had
Q10: How many grams of Ba(OH)2 are needed
Q13: The cylinder shown contains 0.79 moles of
Q13: Which of the following statements is incorrect?
A)If
Q15: Which of the following correctly applies to
Q18: You are required to prepare a 3.50%
Q21: What volume of water,which has a density
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents