The structural formula for glycerine is shown below.Compare the intermolecular forces in glycerine with those in n-hexane,C6H14,in which all carbons are in a continuous chain.Which of the following statements is true? 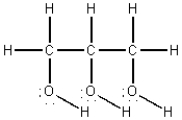
A) The principal intermolecular forces in glycerine are induced dipole forces; in hexane,hydrogen bonds
B) Hydrogen bonding is present in glycerine,but not in hexane
C) Hydrogen bonding is present in both compounds
D) Both compounds exhibit dipole-dipole forces
E) Induced dipole forces are present in neither compound
Correct Answer:
Verified
Q1: Acetone,a highly volatile liquid,is placed in a
Q4: Identify the most correct statement comparing intermolecular
Q6: Which of the following statements is correct?
A)Intermolecular
Q8: The total pressure exerted by the oxygen
Q9: Which of the following properties of liquids
Q10: With all other factors being equal,which of
Q14: Hydrogen gas can be collected by water
Q15: Examine the structure shown below. 
Q16: In a gas mixture the partial pressure
Q20: The kinetic molecular theory as applied to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents