Determine the STP volume of sulfur hexafluoride that will be produced from the reaction of 25.0 kg of sulfur in the reaction S( 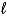 ) + 3 F2(g) SF6(g) .
) + 3 F2(g) SF6(g) .
A) 0.0348 kL
B) 34.8 kL
C) 0.0175 kL
D) 17.7 kL
E) 1.75 *104 kL
Correct Answer:
Verified
Q35: What is the volume of 0.0775 mole
Q36: What is the molar volume of CO2
Q37: Consider the reaction CH4(g)+ H2O(g)
Q38: The molar volume of a gas at
Q39: Which of the following statements is incorrect?
i.The
Q40: Consider the reaction 2 NH3(g)+ CO2(g)
Q41: A 399 mL sample of ethane
Q42: Consider the reaction CO(g)+ 2 H2(g)
Q43: Liquid butane burns according to the
Q44: If 26.0 liters of methane,CH4,measured at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents