Consider the following general wedge-and-dash diagrams. (i) 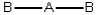 (ii)
(ii) 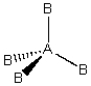
(iii) 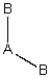
Each consists of a central atom bonded to 2 or 4 terminal atoms that are identical to one another. The central atom is different from the terminal atoms. Which of these molecules is/are polar?
A) i only
B) ii only
C) iii only
D) ii and iii
E) i,ii,and iii
Correct Answer:
Verified
Q25: Consider the following model. Q25: Which of the following has a trigonal Q27: Which of the following molecular models has Q28: Which of the following best describes a Q30: Which of the following is better classified Q31: Which of the following has a(n)bent (angular)molecular Q31: Which of the following molecules is/are polar? Q36: Which of the following molecules is/are nonpolar? Q37: Which of the following molecular geometries yields Q39: Which of the following is considered to![]()
(i)
i.NH3
ii.H2O
iii.HCl
iv.CH4
v.CH2Cl2
A)i
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents