Consider the following periodic table. 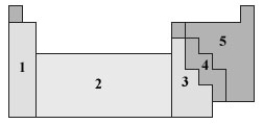 In which of the numbered sections will elements form anions that are isoelectronic with a noble gas?
In which of the numbered sections will elements form anions that are isoelectronic with a noble gas?
A) 1
B) 2
C) 3
D) 4
E) 5
Correct Answer:
Verified
Q4: How does a phosphorus atom achieve an
Q4: Consider the compound represented below. 
Q7: Which of the following has/have the electron
Q8: How is the bond in an F2
Q9: What is a lone pair in a
Q11: Why does the stability of a noble
Q12: A Lewis formula or Lewis diagram is
Q12: The general term to indicate a negatively
Q16: Which of the following ions is/are isoelectronic
Q18: What is the electron configuration of an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents