How many single,double,and triple bonds are in the following molecule? 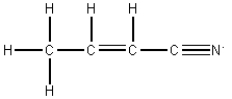 # single bonds # double bonds # triple bonds
# single bonds # double bonds # triple bonds
A) 5 1 1
B) 7 1 1
C) 8 1 1
D) 9 1 1
E) 10 1 0
Correct Answer:
Verified
Q21: Among the following bonds,which would be the
Q22: Given the following electronegativities: H = 2.1,F
Q23: Which is the correct procedure to rank
Q29: Which bond is completely nonpolar?
A)F-F
B)H-F
C)C-N
D)H-O
E)C-H
Q30: Which of the following is the best
Q30: Which of the following correctly lists the
Q31: Which element will act as the negative
Q32: In deciding whether a bond is polar
Q34: Consider the following image of two atoms
Q37: Which element will act as the negative
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents