Consider the following thermochemical equation: CaO(s) + H2O( 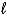 ) Ca(OH) 2(s) H = -66.5 kJ Which of the following statements is/are correct?
) Ca(OH) 2(s) H = -66.5 kJ Which of the following statements is/are correct?
(i) The reaction is exothermic
(ii) The reaction releases 66.5 kJ per gram of Ca(OH) 2(s) formed
(iii) Alternatively,the H term could be added to the product side of the equation
A) i only
B) ii only
C) i and ii
D) i and iii
E) i,ii,and iii
Correct Answer:
Verified
Q28: A typical diet provides about 2000 food
Q29: A typical candy bar contains 281 food
Q30: When 1.00 g of C6H12O6(s)is formed
Q31: If the reaction N2 + 3
Q32: The thermochemical equation describing the heat
Q34: Convert 357 J to kcal.
A)1.49 kcal
B)1.49 *
Q35: The decomposition of potassium chlorate is
Q36: Aqueous HCl is added to an
Q37: A chemist combines hydrochloric acid with a
Q38: Calculate the mass of Na2O that
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents