In the presence of ultraviolet light, the "inert" gas xenon (Xe) will react with fluorine (F2) gas to produce solid XeF4. What is the equilibrium expression for this reaction?
A) 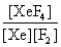
B) 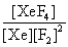
C) 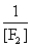
D) 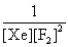
E) 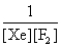
Correct Answer:
Verified
Q31: Consider the reaction system CH4(g) + 2O2(g)
Q32: Consider the reaction system CH4(g) + 2O2(g)
Q33: Consider the following equilibrium: 2H2(g) + X2(g)
Q34: Given the equation A(g) Q35: Consider the reaction 2H2(g) + O2(g) Q37: Consider the reaction system CH4(g) + 2O2(g) Q38: Consider a system of four gases. The Q39: Consider the reaction system CH4(g) + 2O2(g) Q40: Given the equation A(g) Q41: The solubility of BaCO3(s) in water at![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents