Calculate the concentration of the copper ion is a saturated solution of copper(I) cyanide, CuCN (Ksp = 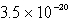 ) .
) .
A) 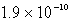
B) 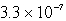
C) 
D) 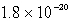
E) none of these
Correct Answer:
Verified
Q37: Consider the reaction system CH4(g) + 2O2(g)
Q38: Consider a system of four gases. The
Q39: Consider the reaction system CH4(g) + 2O2(g)
Q40: Given the equation A(g) Q41: The solubility of BaCO3(s) in water at Q43: The solubility of ZnS(s) in water at Q44: Write the balanced equation for the dissolving Q45: Write the balanced equation for the dissolving Q46: Lithium carbonate, Li2CO3 has a Ksp of Q47: Write the balanced equation for the dissolving![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents