You have 3.00 L of a 3.00 M solution of NaCl(aq) called solution A. You also have 2.00 L of a 1.84 M solution of AgNO3(aq) called solution B. You mix these solutions together, making solution C. Calculate the concentration (in M) of 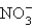 ions in solution C.
ions in solution C.
A) 0 M
B) 0.736 M
C) 1.06 M
D) 1.84 M
E) 1.80 M
Correct Answer:
Verified
Q45: Calculate the volume of 0.164 M HNO3
Q46: What volume of 17.4 M H2SO4 is
Q47: What volume of 12.0 M nitric acid
Q48: You have 3.00 L of a 3.77
Q49: Magnesium metal reacts with hydrochloric acid to
Q51: If you mix 40.0 mL of a
Q52: What is the normality of 1.00 L
Q53: In the following acid-base neutralization, 1.61
Q54: Determine the normality of a base if
Q55: Calculate the volume of 0.581 M KOH
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents