The molar heat of fusion of water is 6.02 kJ/mol. Calculate the energy required to melt 44.3 g of water.
A) 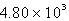 kJ
kJ
B) 267 kJ
C) 2.46 kJ
D) 133 kJ
E) 14.8 kJ
Correct Answer:
Verified
Q4: The bonds between hydrogen and oxygen in
Q5: At 1 atm of pressure and a
Q6: Which of the following has the lowest
Q7: The molar heat of fusion of water
Q8: At 1 atm pressure, liquid water always
Q10: The molar heats of fusion of water
Q11: Calculate the quantity of energy required to
Q12: The specific heat capacity of liquid water
Q13: The molar heat of fusion of water
Q14: The specific heat capacity of liquid water
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents