You have a partially filled party balloon with 2.00 g of helium gas. You then add 3.00 g of hydrogen gas to the balloon. Assuming constant temperature and pressure, how many times bigger is the party balloon - comparing before and after the hydrogen gas has been added. Choose the best formula to use to solve this problem. (V = volume of the gas, n = moles of the gas, m = mass of the gas, P = pressure of the gas.)
A) 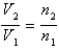
B) 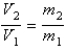
C) 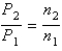
D) 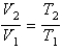
E) 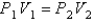
Correct Answer:
Verified
Q32: You are holding two balloons of the
Q33: Which of the following will give a
Q34: A sample of a gas in a
Q35: Which of the following statements are true
Q36: If temperature and pressure are held constant,
Q38: You transfer a sample of gas at
Q39: You have a certain mass of helium
Q40: Gaseous chlorine is held in two separate
Q41: Zinc metal is added to hydrochloric acid
Q42: A gas occupies 42.6 L at 2.00
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents