A compressed gas cylinder, at 137 atm and 23oC, is in a room where a fire occurs. The fire raises the temperature of the gas to 475oC. What is the new pressure in the cylinder?
A)  atm
atm
B) 3.30 atm
C) 85.4 atm
D) 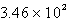 atm
atm
E) 2.16 atm
Correct Answer:
Verified
Q62: How many liters of HCl(g) measured at
Q63: You place 15.0 g of nitrogen
Q64: You transfer a gas at 25oC from
Q65: Which of the following statements is true
Q66: How many moles of O2(g) are needed
Q68: When acetylene gas, C2H2, burns, how many
Q69: A sample of argon gas occupies a
Q70: A sample of oxygen gas (O2) has
Q71: Solid carbon dioxide is placed into a
Q72: Hydrogen peroxide decomposes to form water and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents