Consider the drawings below: 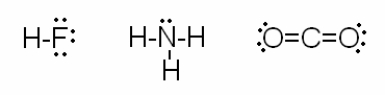 Which of the following statements are true? I. The electrons in each molecule tend to be attracted to the most electronegative element.
Which of the following statements are true? I. The electrons in each molecule tend to be attracted to the most electronegative element.
II) Each molecular drawing follows the localized electron model.
III) Both HF and CO2 are linear molecules and therefore nonpolar.
IV) The bond angles of NH3 are slightly less than 109.5o because the lone pair compresses the angles between the bonding pairs.
A) I, III, IV
B) I, II, IV
C) I, II, III
D) II, IV
E) All of the above statements (I - IV) are correct.
Correct Answer:
Verified
Q9: A bond is a force that holds
Q10: The greater the difference in electronegativity between
Q11: CH4 has ionic bonds.
Q12: Covalent bonding occurs when electrons are shared
Q12: Which of the following bonds would be
Q14: Would OCl2 be classified as ionic or
Q15: Would NaBr be classified as ionic or
Q16: N2 is an example of a covalent
Q17: Rank the following bonds from least polar
Q18: Would NH3 be classified as ionic or
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents