How much energy will be needed to heat 69.7 gal of water from 22.0°C to 110.0°C? (Note that 1.00 gal weighs 3.77 kg and that water has a specific heat capacity of 4.184 J/g·°C.)
A) 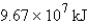
B) 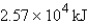
C) 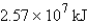
D) 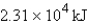
E) 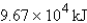
Correct Answer:
Verified
Q8: Calculate the heat given off when 159.7
Q9: Assume that 491.8 J of heat is
Q10: Which is larger, one calorie or one
Q11: A system absorbs 159 kJ of heat,
Q12: How many joules of energy would be
Q14: The specific heat capacity of silver is
Q15: SI units for specific heat capacity are
A)
Q16: The quantity of heat required to change
Q17: A 6.75-g sample of gold (specific heat
Q18: Express ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents