For the reaction 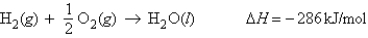 Calculate the enthalpy change when 2.80 g of water is produced.
Calculate the enthalpy change when 2.80 g of water is produced.
A) 102 kJ
B) 801 kJ
C) -801 kJ
D) 44.4 kJ
E) -44.4 kJ
Correct Answer:
Verified
Q19: Calculate Q20: A 4.60-g sample of iron is heated Q21: Energy can be classified as either potential Q22: The energy gained by the surroundings must Q23: _ is a measure of the random Q25: Determine the enthalpy change when 19.4 g Q26: Perform the indicated conversion: 1.345 kcal =_ Q27: Which of the following processes is endothermic? Q28: The specific heat capacity of gold is Q29: For the reaction ![]()
A)![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents