Consider the equation: 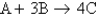 . If 3.0 moles of A is reacted with 6.0 moles of B, which of the following is true after the reaction is complete?
. If 3.0 moles of A is reacted with 6.0 moles of B, which of the following is true after the reaction is complete?
A) A is the leftover reactant because you need only 2 moles of A and have 3.
B) A is the leftover reactant because for every 1 mole of A, 4 moles of C are produced.
C) B is the leftover reactant because you have more moles of B than A.
D) B is the leftover reactant because 3 moles of B react with every 1 mole of A.
E) Neither reactant is leftover.
Correct Answer:
Verified
Q54: Determine the mass of CO2 produced when
Q55: Consider the reaction Q56: How many moles of SbCl3 is formed Q57: Fe3O4 reacts with CO according to the Q58: Fe3O4 reacts with CO according to the Q60: Fe2O3 (molar mass = 159.7 g/mol) reacts Q61: Consider a reaction in which two reactants Q62: Consider the equation: A + 4B Q63: Consider the following unbalanced equation: Q64: Consider the following reaction at 1.10 atm![]()

Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents