Ammonia reacts with oxygen to form nitrogen dioxide and water according to the following equation: 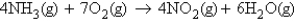 You react ammonia and oxygen, and at the end of the experiment you find that you produced 23.0 g of water and have 8.52 g of ammonia left over. Determine the mass of oxygen reacted.
You react ammonia and oxygen, and at the end of the experiment you find that you produced 23.0 g of water and have 8.52 g of ammonia left over. Determine the mass of oxygen reacted.
A) 35.0 g O2
B) 17.7 g O2
C) 40.9 g O2
D) 71.5 g O2
E) 47.7 g O2
Correct Answer:
Verified
Q72: Consider the following unbalanced equation: 
Q73: In the reaction between CO and Fe3O4,
Q74: Equal masses of hydrogen gas and oxygen
Q75: Consider the following unbalanced equation: 
Q76: Consider that calcium metal reacts with oxygen
Q78: You react 25.0 g hydrogen gas with
Q79: Consider the reaction of magnesium metal with
Q80: Reacting 3.00 mol nitrogen gas with 3.50
Q81: Consider the reaction of magnesium metal with
Q82: Consider the reaction of magnesium metal with
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents