Consider the following reaction at 1.10 atm and 19°C: 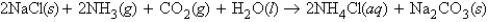 0.228 mol of sodium chloride, 3.03 L of ammonia, 2.00 L of carbon dioxide, and an unlimited amount of water react to form aqueous ammonium chloride and solid sodium bicarbonate. How many moles of ammonium chloride are formed in the reaction?
0.228 mol of sodium chloride, 3.03 L of ammonia, 2.00 L of carbon dioxide, and an unlimited amount of water react to form aqueous ammonium chloride and solid sodium bicarbonate. How many moles of ammonium chloride are formed in the reaction?
A) 0.139 mol
B) 0.278 mol
C) 0.228 mol
D) 6.06 mol
E) 0.0918 mol
Correct Answer:
Verified
Q59: Consider the equation: Q60: Fe2O3 (molar mass = 159.7 g/mol) reacts Q61: Consider a reaction in which two reactants Q62: Consider the equation: A + 4B Q63: Consider the following unbalanced equation: ![]()

Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents