Multiple Choice
One mole of something consists of ____________ units of that substance.
A) 12.01
B) 1.008
C) 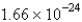
D) 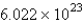
E) 32.00
Correct Answer:
Verified
Related Questions
Q2: You have 12.6 g of an unknown
Q3: Calculate the mass of 23.7 moles of
Q4: One atomic mass unit (amu) is the
Q5: A 1.50-mol sample of Zr represents how
Q6: A 3.37-mol sample of aluminum represents how
Q8: A 30.5-g sample of Ca contains how
Q9: How many moles of Ca atoms are
Q10: How many atoms of calcium are present
Q11: A 2.35-mole sample of H2O2 weighs
A) 79.9
Q12: The formula of a compound that expresses
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents