Multiple Choice
Calculate the mass of 3.663 moles of silver nitrate.
A) 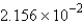 g
g
B) 622.4 g
C) 46.4 g
D) 563.8 g
E) none of these
Correct Answer:
Verified
Related Questions
Q57: Calculate the number of moles of water
Q58: Convert: Q59: Calculate the mass of 5.986 mol of Q60: A 84.9-g sample of SO2 contains how Q61: Consider separate 100.0-g samples of each of Q63: Calculate the percentage composition (by mass) of Q64: Calculate the number of moles of Cl2 Q65: A hydrocarbon has the formula C5H12. What Q66: A sample with Q67: Calculate the number of molecules in 0.000400![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents