A 60.2-mL sample of Hg (density = 13.6 g/mL) contains how many atoms of Hg?
A) 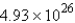 atoms
atoms
B) 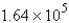 atoms
atoms
C) 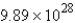 atoms
atoms
D) 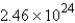 atoms
atoms
E) none of these
Correct Answer:
Verified
Q72: The mole can be defined as the
Q73: How many moles of Al2O3 are present
Q74: A gaseous compound containing carbon and hydrogen
Q75: Calculate the mass of 0.314 mol of
Q76: Calculate the number of moles of CH4
Q78: Calculate the mass of sulfur in 1.74
Q79: Calculate the number of moles in 2.28
Q80: A gaseous compound containing carbon and hydrogen
Q81: The mass percent of chlorine in BaCl2
Q82: Calculate the empirical formula of a compound
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents